Indicate How Many Unpaired Electrons Each Atom Has.
The number of unpaired electrons are 0. V 4 unpaired electrons.
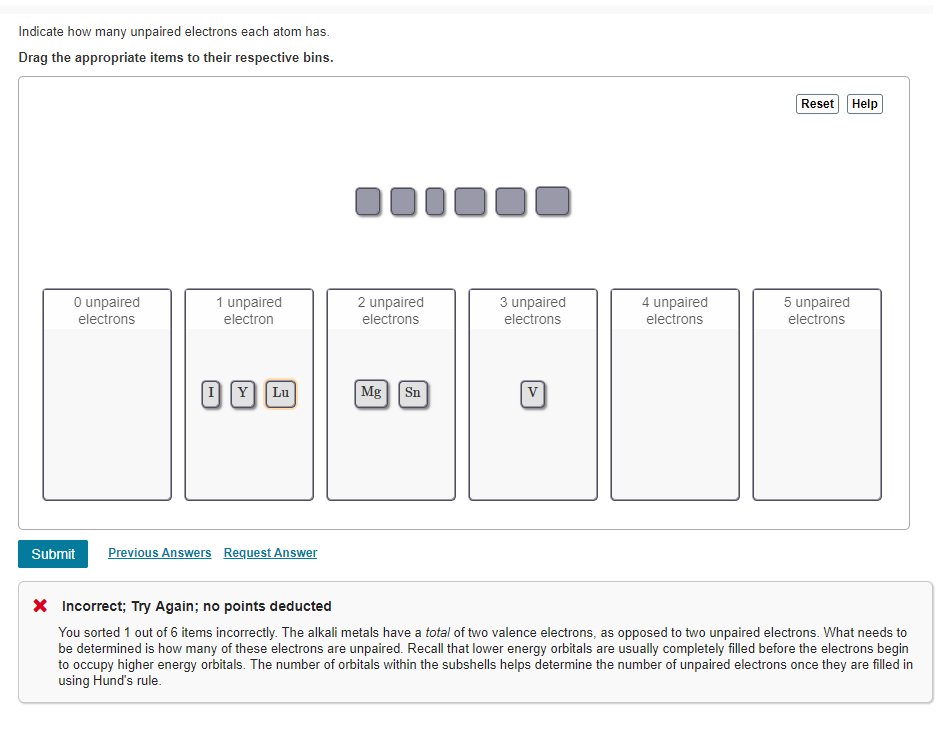
Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com
Step 1 1 of 2.
. Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons each has. Since all orbitals are fully occupied there are no unpaired electrons in krypton. During compound formation the yttrium atom donates three electrons to another atom and the oxidation state of yttrium is 3.
Ca 1 unpaired electron. No of unpaired electrons 4 e Krypton Kr. 1 How Many Of The Following Elements Have 2 Unpaired Electrons In The Ground State.
Drag the appropriate items to their respective bins. 10 What element has the electron configuration 1s 2s 2p 3s 3p. In chemistry an unpaired electron is an electron that occupies an orbital of an atom singly rather than as part of an electron pair.
So the electron configuration is 1s2. 3 How many unpaired electrons are there in the ground state of each atom. Sn 3 unpaired electrons.
Here all orbitals are fully occupied there are no unpaired electrons in Kr. 1s2 2s2 2p6 3s2 3p6 4s2 3d6. No of unpaired electrons 6 since 1 electron is to be added to 4s 5 electron to be added to 3d orbital.
15 How many protons electrons and neutrons are there in potassium. Br Y Lu 2 unpaired electrons. E Krypton Kr atomic number 36.
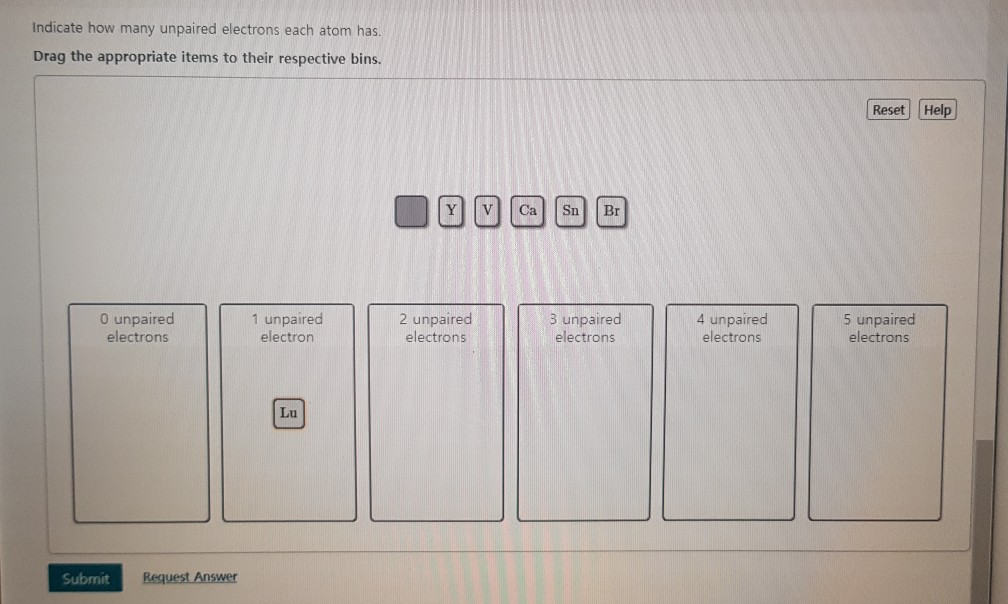
4 Does SN have 2 unpaired electrons in the ground state. Lutetium atoms have 71 electrons and the shell structure is 28. Oct 18 2016 Well nitrogens atomic number is 7 so it has 5 valence electrons two in the 2s orbital and three in the 2p orbitals and 2 core electrons two in the 1s orbital.
The condensed electronic configuration is. For example the element P has an Atomic Mass of 15. 13 Which atom has only two unpaired electrons in its ground state.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. In the element bromine Br there is only 1 unpaired electron. An orbital is made up of 2 electrons and any orbitals left with only 1 electron is considered unpaired.
As Noble gases have no unpaired electrons the only. 5 How many unpaired electrons does CU have in ground state. D Iron Fe atomic number 26.
Therefore the valency of the yttrium element is 3. Chemistry Electron Configuration Electron Configuration 1 Answer Truong-Son N. 13 Which element has an equal number of electron pairs and of unpaired electrons.
Number of unpaired electrons 4. None 5 unpaired electrons. TextbfUnpaired electrons Unpaired electrons.
Chemistry questions and answers. 15 Does nickel have 10 valence electrons. Ga 1s2 2s2 2p2 2p2 2p2 3s2 3p2 3p2 3p2 3d2 3d2 3d2 3d2 3d2 4s2 4p1 or 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1.
N e3s2 N e 3 s 2. C Te Hf Si. 9 Which atom in the ground state has 5 electrons in its outer level.
How many unpaired electrons are in a N atom. Valency and valence electrons of yttrium Y. See full answer below.
8 Which atom in the ground state has two unpaired electrons. Identify which sets are valid and classify the others by the rule or principle that is violated. It has 7 valence electrons so 3 pairs plus an unpaired electron.
Drag the appropriate items to their respective bins. The elements Ga Ge As Se and Br lie in the same period in the periodic table. Ne 3 s 2 3s2 3 s 2.
Also to know is how many unpaired electrons does each element have. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. 2 Which element has exactly 2 unpaired electrons in its ground state.
Condensed Electron Configurations and Unpaired Electrons. 14 How many electrons are there in the atom. 14 How many neutrons protons and electrons do potassium have.
6 How many electrons does a Co atom have in its 3d subshell number of electrons 3d electrons How many of those electrons are unpaired number of unpaired electrons. 7 What is the electron configuration of Co 3. Write the electron configuration expected for the ground-state atoms of these elements and predict how many unpaired electrons if any each atom has.
TextbfElectronic configuration Electronic configuration. Indicate how many unpaired electrons each atom has. Number of unpaired electrons 6.
The following sets of quantum numbers listed in the order n ℓ mℓ and ms were written for the last electrons added to an atom. The number of unpaired electrons in the last orbit of an element is the valency of that element. Argon 1s2 2s2 2p2 2p2 2p2 3s2 3p2 3p2 3p2 Ga Ar 3d2 3d2 3d2 3d2 3d2 4s2 4p1 or Ar 3d10 4s2 4p1.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Reset Help G L U ME Y O unpaired electrons 1 unpaired electron 2 unpaired electrons 3 unpaired electrons 4 unpaired electrons 5 unpaired electrons. See answer 1 Best Answer.
Indicate how many unpaired electrons each atom has.
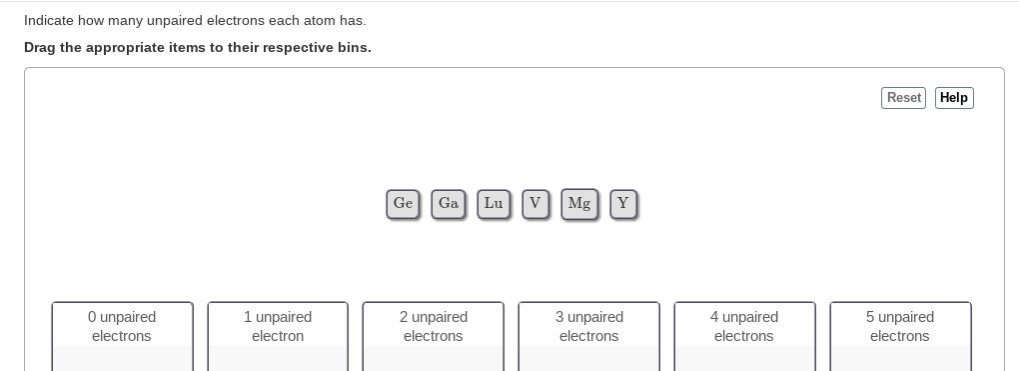
Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com

Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com
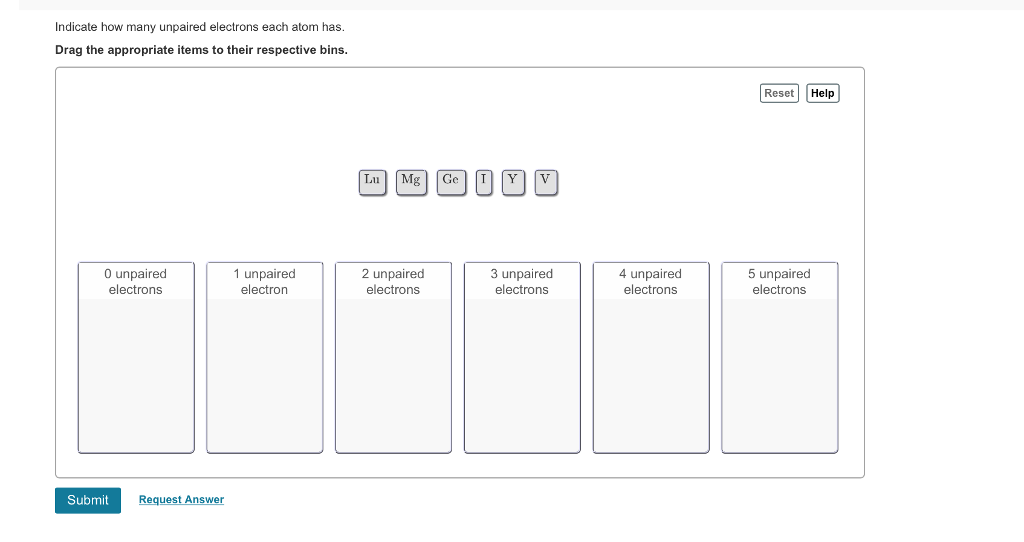
Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com
Belum ada Komentar untuk "Indicate How Many Unpaired Electrons Each Atom Has."
Posting Komentar