Boron Occurs in Nature in the Form of Two Isotopes
B-10 is used in the form of boric acid as a chemical shim in pressurized water reactors while in the form of sodium pentaborate it is used for standby liquid control systems in boiling water reactors. Boron occurs in nature in the form of two isotopes 5B 115B 10 in the ratio of 81 and 19 respectively calculate its average atomic mass.

Example 11 Boron Occurs In Nature In The Form Of Two Isotopes Having Atomic Mass 10 And 11 What Are The Percentage Abundances Of Two Isotopes In A Sample Of Boron Having
B-11 can be used as a neutron reflector.

. Boron naturally occurs in two isotopic forms. Calculate the percentage abundances of two isotopes respectively in a sample having atomic mass 1080. Two isotopes of boron occur naturally with 105B at 1990 1001 amu and 115B at 8010 1101 amu.
The average atomic mass of boron is 1081. And 1081 u is a lot closer to 11u than it is to 10u so there must be more of boron-11. Calculate the atomic mass for boron using the.
What are the percentage abundances of these isotopes in a simple a boron having average atomic mass of 108 are. Naturally occuring boron has two isotopes of masses 10 amu and 11 amu. Boron occurs in nature in the form of two isotopes 115 and B and 105 in ratio of 81 and 19 respectively.
They have different numbers of protons and different mass numbers. Boron occurs naturally as two isotopes. Boron occurs in nature in the form of two isotopes 115 and B and 105 in ratio of 81 and 19 respectively.
Given atomic no of B 5u 81 is the relative abundance of 11 B 5 isotope in nature and 19 is the relative abundance of 10 B 5 isotope in nature. Both isotopes of Boron B-10 and B-11 are used extensively in the nuclear industry. What is the difference between these isotopes.
Calculate the atomic mass for boron using the weighted average mass method. Boron occurs in nature in the form of two isotopes having atomic mass 10 and 11. Calculate the percentage abundances of two isotopes respectively in a sample having atomic mass 1080.
They have different numbers of neutrons and different charges. The natural abundance of Boron-10 is 199 with 10013 amu and Boron-11 is 801 with 11009 amu. Two isotopes of boron occur naturally with 105B at 1990 1001 amu and 115B at 8010 1101 amu.
Boron-11 The atomic mass of boron is 1081 u. In order to calculate the average atomic weight. Only two isotopes of boron B occur in nature.
Calculate its average atomic mass. Complete the table by computing the relative atomic mass of 11 B to four significant figures taking the tabulated relative atomic mass of natural boron as 10811. The atomic weight of Boron is 11.
Boron occurs in nature in the form of two isotopes class 11 chemistry CBSE. Boron occurs in nature in the form of two isotopes- 11B and 10B in the ratio of 81 and 19 respectively. To convince you fully we can also do a simple calculation to find the exact proportion of boron-11 using the following formula.
They have different numbers of neutrons and different mass numbers. They have different numbers of electrons and different charges. What is the mass of the other isotope.
The mass difference results in a wide range of δ 11 B values which are defined as a fractional difference between the 11 B and 10 B and traditionally expressed in parts per thousand in natural waters ranging from 16 to 59. Calculate its average atomic mass. Boron occurs in nature in the form of two isotopes having atomic mass 10 and 11.
Their atomic masses and abundances are given in the following table. Boron occurs in nature in the form of two isotopes having atomic masses 10 and 11. 10 ux11 u1-x1001081u Where u is the unit for atomic mass and x is the proportion of.
How to solve thispls helpBoron occurs in nature in the form of two isotopes 11 B 5 and 10 B 5 in the ratio of 81 and 19 respectively. The more common isotope is 11b atomic mass 1101 amu which is 8000 abundant. Boron occurs naturally in two isotopic forms.
Calculate the percentage of each isotope if the average atomic mass of boron is. Naturally occuring boron has two isotopes of masses 10 amu and 11 amu. Boron has two naturally occurring and stable isotopes 11 B 801 and 10 B 199.
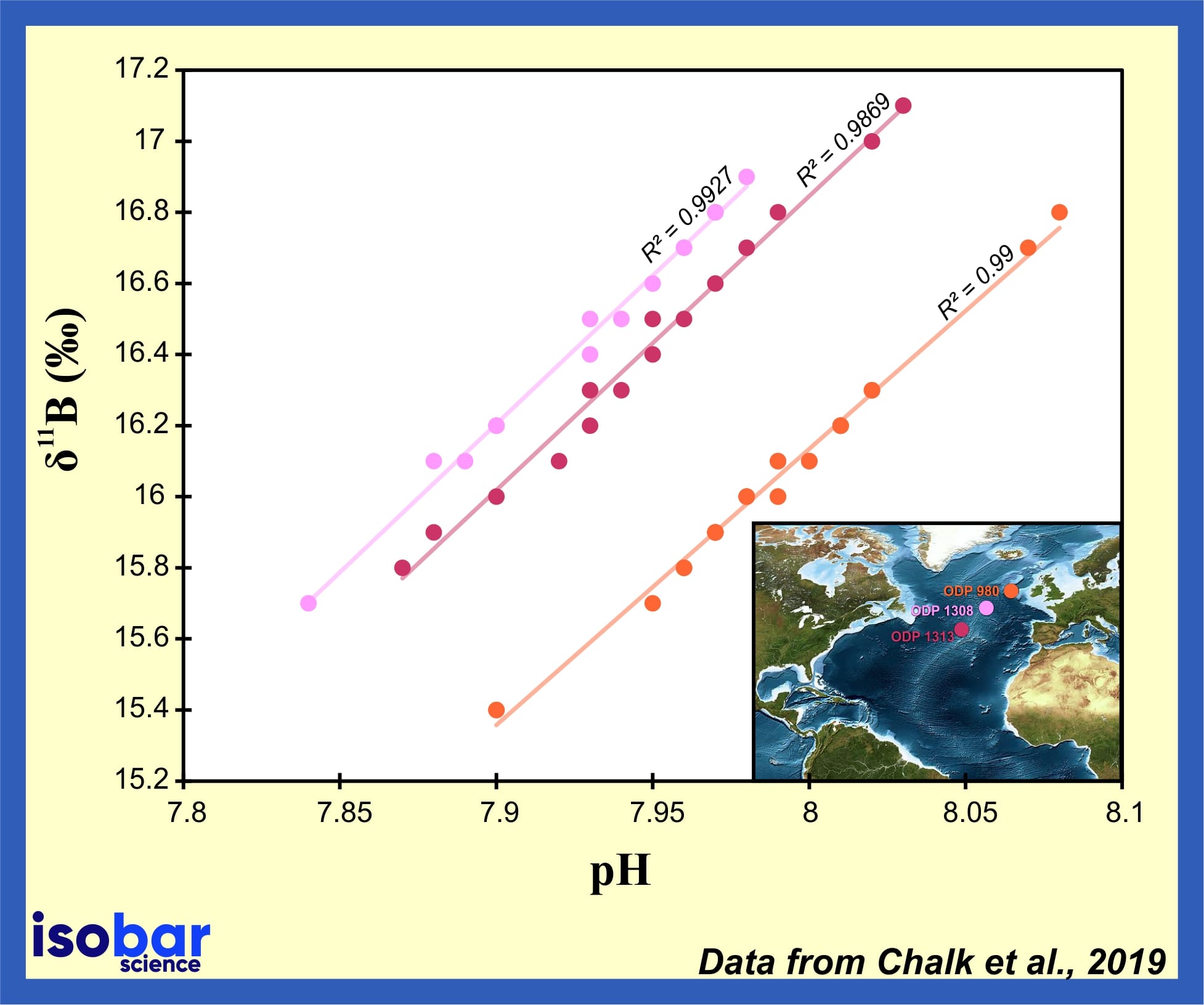
Boron Isotopes Geochemistry Isobar Science

U Example 1boron Occurs In Nature In The Form Of Two Isotopes Having Atomic Mass 10 And 11 What Are The Percentage Abundances Of Two Isotopes In A Sample Of Boron Having
Belum ada Komentar untuk "Boron Occurs in Nature in the Form of Two Isotopes"
Posting Komentar